
Zeitschrift für Naturforschung B 62 (10): 1227. "Sr143: Eine Zintl-Phase mit isolierten 4–- und 8–-Anionen / Sr1423: A Zintl Phase with Isolated 4–- and 8– Anions"] (in de). Al(−2) has been observed in Sr1423, see Wemdorff, Marco Röhr, Caroline (2007).
 Negative oxidation states of p-block metals (Al, Ga, In, Sn, Tl, Pb, Bi, Po) and metalloids (Si, Ge, As, Sb, Te, At) may occur in Zintl phases, see:, p. Bibcode: 1964Natur.202.383T. , and in dialanes (R2Al-AlR2) see Uhl, Werner "Organoelement Compounds Possessing Al-Al, Ga-Ga, In-In, and Tl-Tl Single Bonds" Advances in Organometallic Chemistry Volume 51, 2004, Pages 53–108. "Red (B2Π–A2σ) Band System of Aluminium Monoxide". Al(II) has been observed in aluminium(II) oxide (AlO) see D. "Stable Magnesium(I) Compounds with Mg-Mg Bonds". Low valent magnesium compounds with Mg(I) have been obtained using bulky ligands see Green, S. "Eigenschaften von borreichen Boriden und Scandium-Aluminium-Oxid-Carbiden" (in de). B(−5) has been observed in Al3BC, see Melanie Schroeder. Chemical Structure and Reactivity: An Integrated Approach. B(−1) has been observed in magnesium diboride (MgB2), see James Keeler, Peter Wothers (2014). "Infrared Emission Spectra of BeH and BeD". Be(I) has been observed in beryllium monohydride (BeH) see Shayesteh, A. The periodicity of the oxidation states was one of the pieces of evidence that led Langmuir to adopt the rule.
Negative oxidation states of p-block metals (Al, Ga, In, Sn, Tl, Pb, Bi, Po) and metalloids (Si, Ge, As, Sb, Te, At) may occur in Zintl phases, see:, p. Bibcode: 1964Natur.202.383T. , and in dialanes (R2Al-AlR2) see Uhl, Werner "Organoelement Compounds Possessing Al-Al, Ga-Ga, In-In, and Tl-Tl Single Bonds" Advances in Organometallic Chemistry Volume 51, 2004, Pages 53–108. "Red (B2Π–A2σ) Band System of Aluminium Monoxide". Al(II) has been observed in aluminium(II) oxide (AlO) see D. "Stable Magnesium(I) Compounds with Mg-Mg Bonds". Low valent magnesium compounds with Mg(I) have been obtained using bulky ligands see Green, S. "Eigenschaften von borreichen Boriden und Scandium-Aluminium-Oxid-Carbiden" (in de). B(−5) has been observed in Al3BC, see Melanie Schroeder. Chemical Structure and Reactivity: An Integrated Approach. B(−1) has been observed in magnesium diboride (MgB2), see James Keeler, Peter Wothers (2014). "Infrared Emission Spectra of BeH and BeD". Be(I) has been observed in beryllium monohydride (BeH) see Shayesteh, A. The periodicity of the oxidation states was one of the pieces of evidence that led Langmuir to adopt the rule. 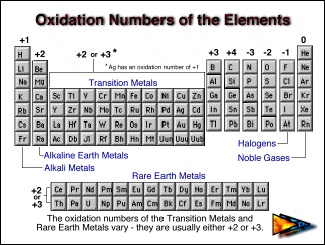
A figure with a similar format (shown below) was used by Irving Langmuir in 1919 in one of the early papers about the octet rule.






 0 kommentar(er)
0 kommentar(er)
